In the early days of our existence (I mean EyesOnALZ, not the Universe 😇) we published a blog series about the project on the SciStarter/DiscoverMag/PLOS Blogs networks.
We're sharing the selected content here! The following post, orig. titled "The Science Behind WeCureALZ * : A Participatory Research Project Tackling Alzheimer’s Disease" was published on April 22, 2016 on Discover Magazine's Citizen Science Salon by Egle Marija Ramanauskaite (me!).
* that was before the WeCureALZ --> EyesOnALZ rebranding).
Alzheimer’s disease (AD) – in a nutshell
Despite the fact that Alzheimer’s is being studied by scientists all around the globe, no clear-cut cause or cure for the disease exists. What we do know, however, is that two main risk factors play a significant part: age and genetics.
The latter is known to cause Familial Alzheimer’s Disease (FAD), which occurs due to a mutation that can be transmitted through generations. FAD is typically associated with early-onset Alzheimer’s, where significant symptoms develop before the age of 60. However, genetically inherited AD is very rare and occurs in less than 5% of all cases. Instead, most Alzheimer’s cases are sporadic with no genetic cause identified to date. These are typically associated with a late onset.
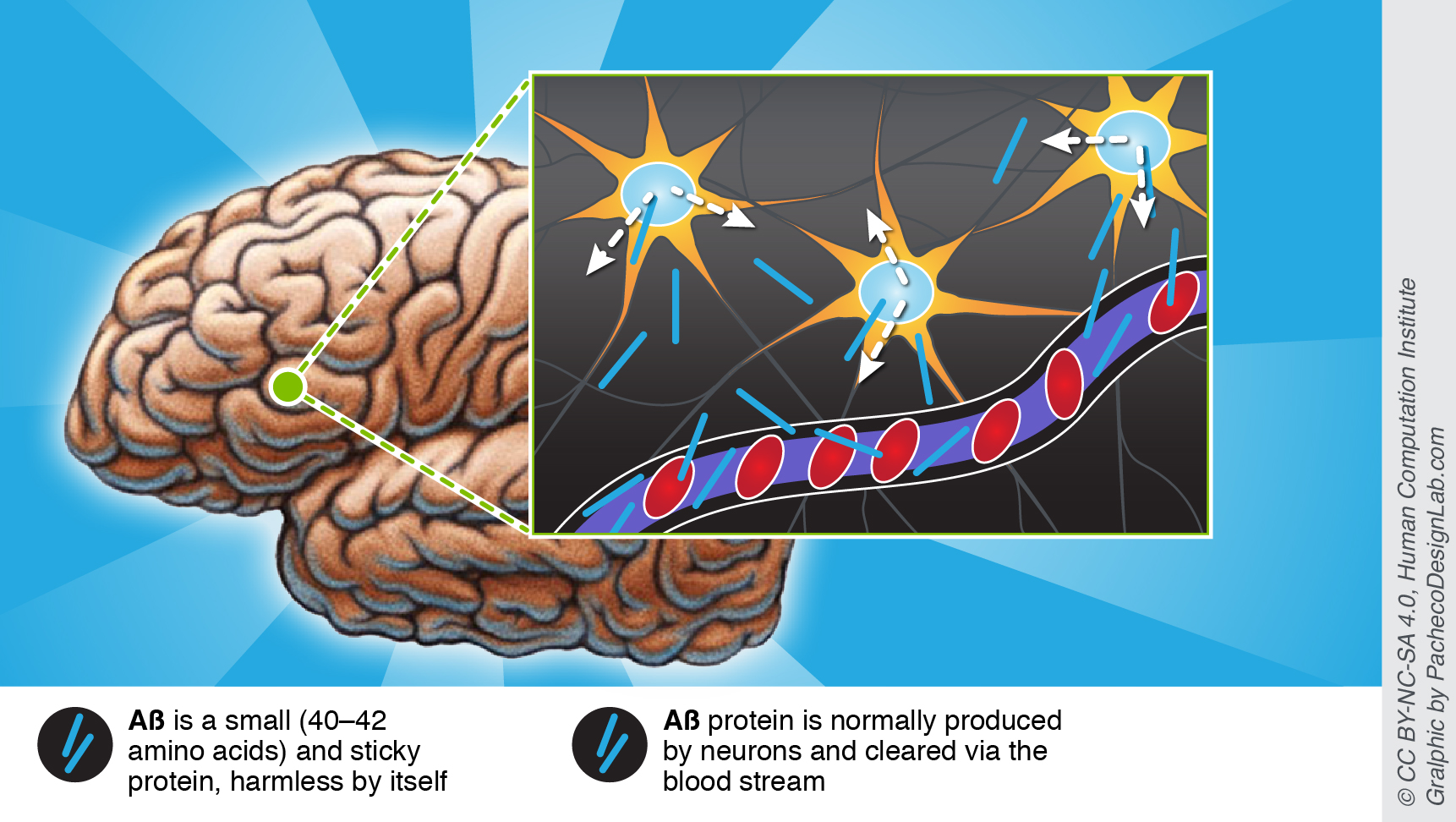
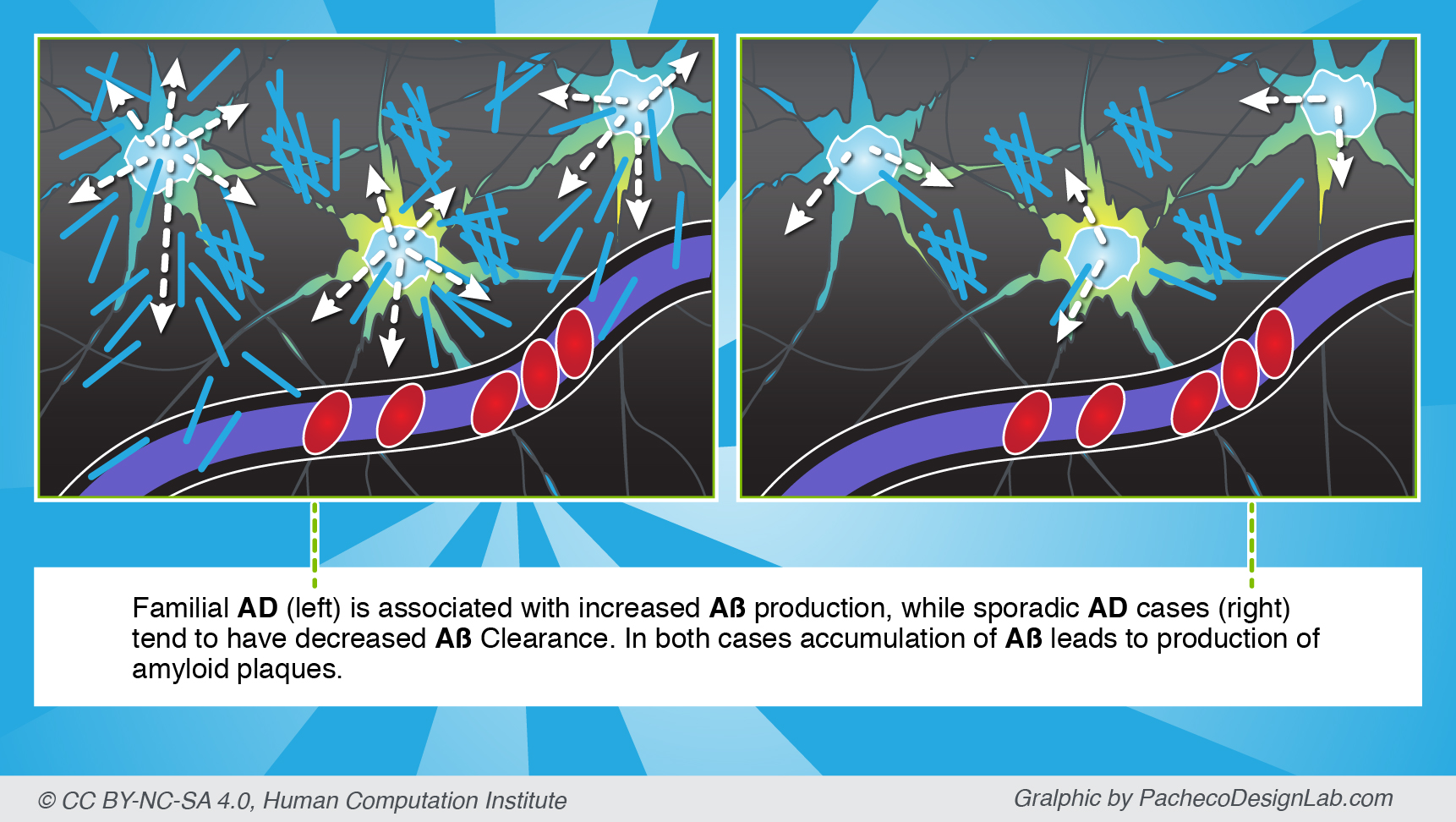
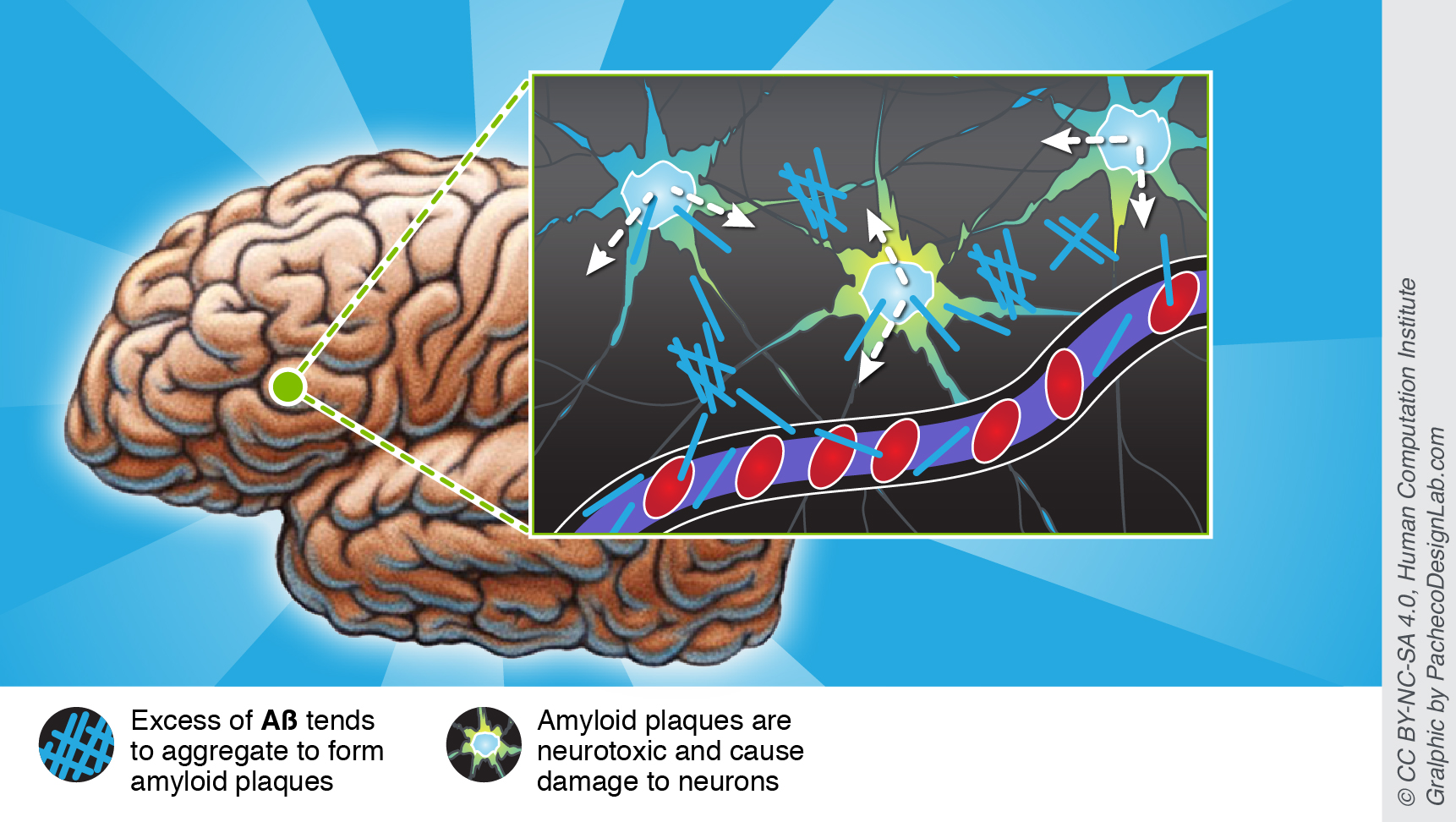
Regardless of the disease type, each has been associated with “sticky” amyloid plaques that are one of the likely culprits of characteristic Alzheimer’s symptoms. Funnily enough, amyloid plaques form from a benign amyloid beta protein (Aβ), which occurs naturally in all humans. Yet, be it genetics or abnormal accumulation of Aβ through time, too much protein leads to amyloid aggregates that are neurotoxic and cause significant damage to the brain. Moreover, aggregates are also harder to clear by natural mechanisms, such as via the blood stream.
Out-of-the-box approach
Recent findings at the Schaffer-Nishimura Lab at Cornell University suggest the best way to reduce the devastating effects of amyloids on the brain might not be through targeting the plaques themselves, but by improving blood flow.
In fact, the researchers have discovered that as many as 2% of tiniest vessels of the brain – the capillaries –are stalled at any one time in the brain of AD-affected mice. That is up to ten times more stalls than normal. Moreover, since one capillary is typically connected to many others, the downstream effects of those stalls can contribute to as much as a 30% decrease in the overall blood flow in the brain!
Similar (10-40%) reduction in blood flow associated with Alzheimer’s has long been recognized in humans too. Importantly, some reduced blood flow occurs even before any symptoms develop. These observations led researchers to believe that decreased blood flow might be one of culprits behind plaque formation at the earliest stages of the disease. It also suggests a possible treatment that if administered early enough, would improve blood flow and prevent Alzheimer’s symptoms from occurring in the first place.
From stalls to amyloids, and back again
As we have already mentioned, Aβ protein is harmless by itself, but it becomes toxic to neurons once it accumulates and forms “sticky” amyloid plaques. Importantly, Aβ clearance depends partially on good circulation in the brain. This means that if blood flow was impaired at the early stages of Alzheimer’s, even less Aβ protein would be cleared, and the accumulation of toxic amyloid aggregates would accelerate.
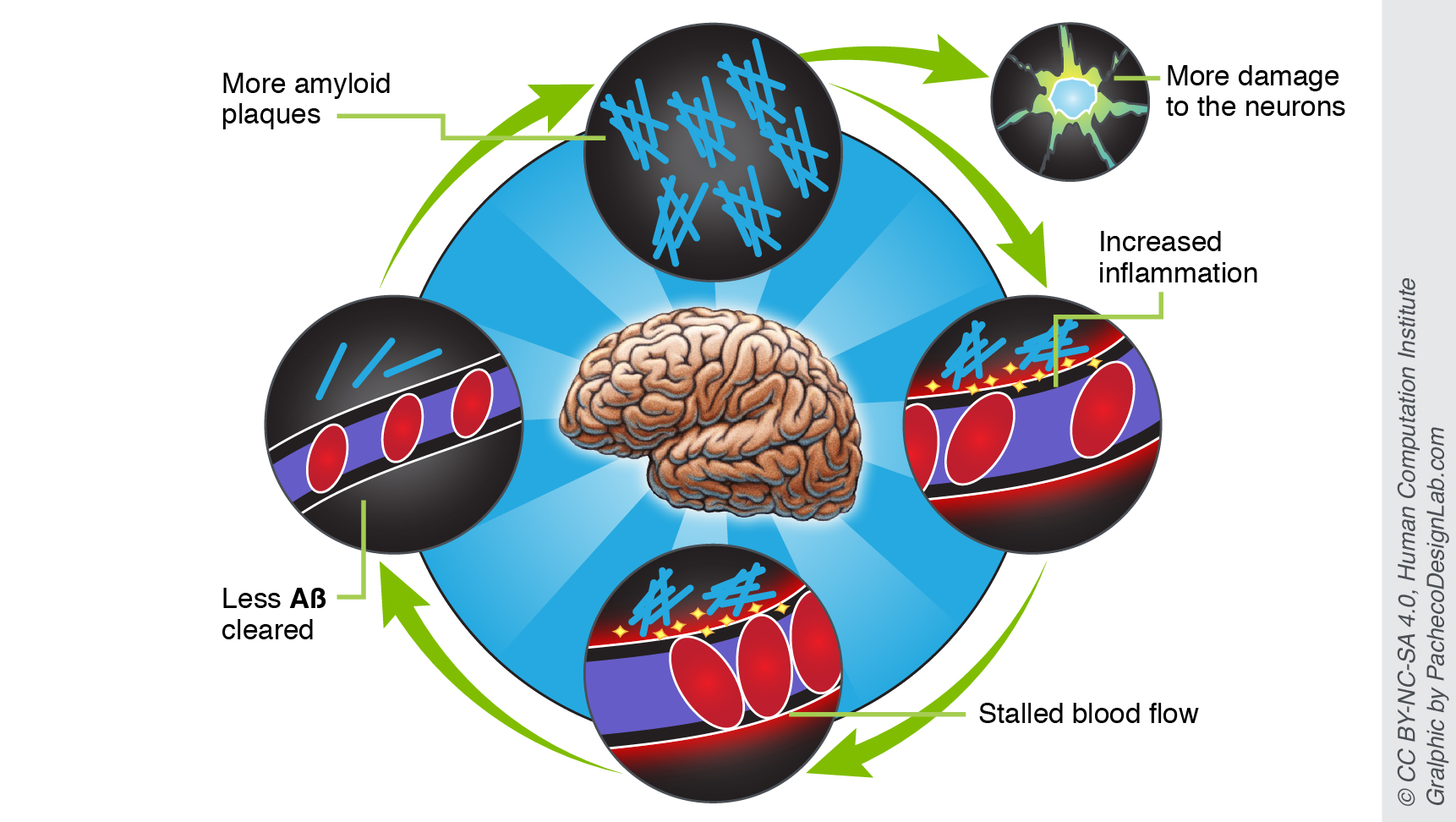
Moreover, far from just hurting the neurons themselves, amyloid plaques also induce a local inflammation, which attracts leukocytes (white blood cells) and encourages them to stick firmly to capillary walls. Such leukocyte “plugs” are responsible for the majority of blood stalls in AD-affected mice.
Therefore, the more that amyloids remain due to decreased blood flow, the more damage they cause and decrease the flow even further.
What can we do?
The findings gathered so far by the Schaffer-Nishimura Lab suggest improving blood flow by, for example, preventing leukocyte from sticking to capillary walls, could help prevent Alzheimer’s symptoms from appearing at all. However, many question marks remain. What are the molecular mechanisms that drive these capillary stalls? Are there any AD-specific steps in this pathway? Can we target them without compromising any other processes in the body? Where do stalls occur with respect to the overall brain architecture, and/or amyloid deposits?
Unfortunately, due to an extremely tedious data analysis step, each research question costs about a year of manual image analysis. At this rate, finding the answers would take decades.
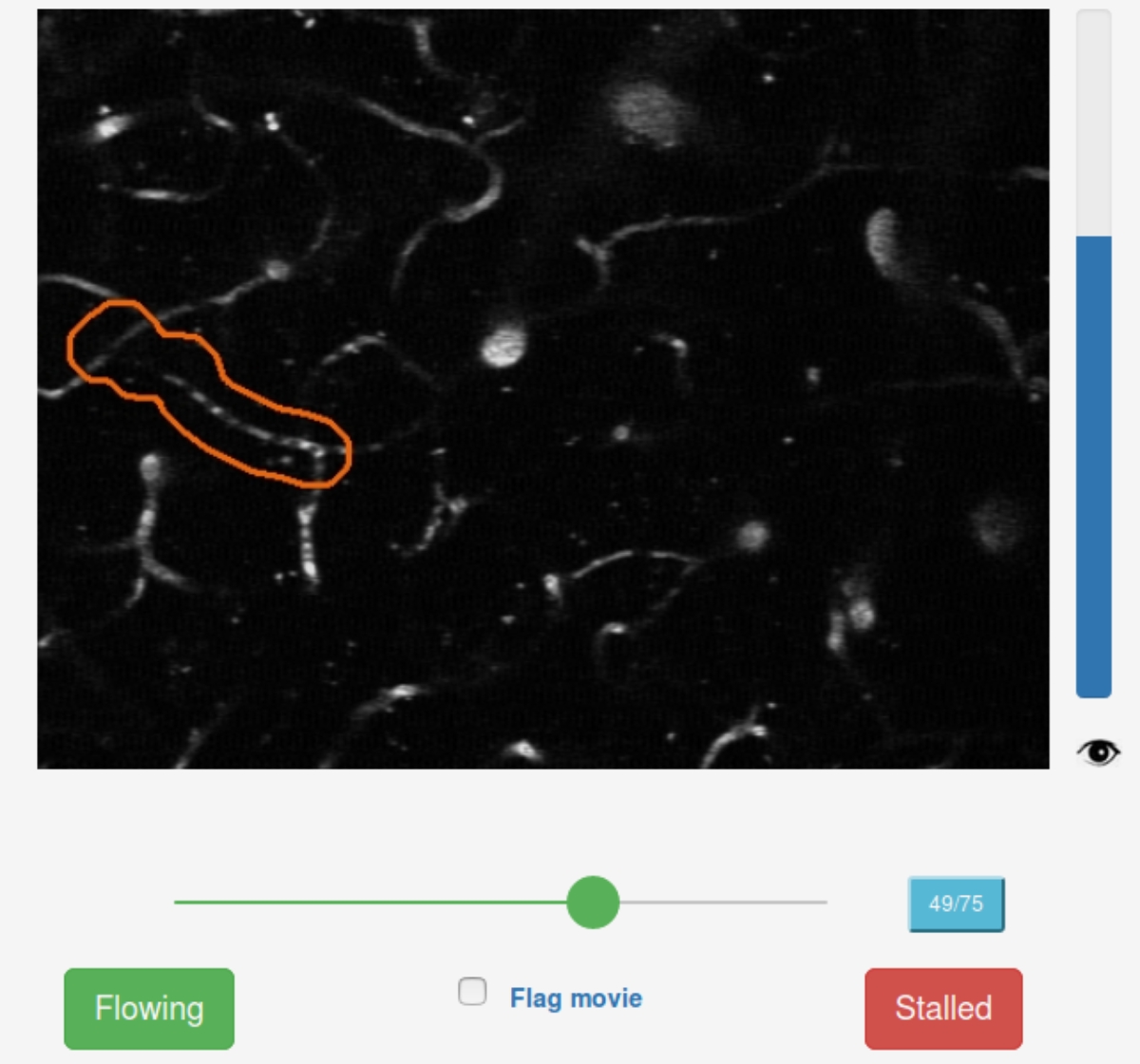
EyesOnALZ, led by the Human Computation Institute, has joined forces with the Schaffer-Nishimura Lab and experienced citizen science teams at Stardust@home and EyeWire to change just that. By allowing citizen scientists (like you!) to look at the same microscopic images that researchers do, EyesOnALZ aims to fast-track the analysis of stall vessels in mice, and understand where and why do they occur.

This means we could solve many of outstanding research questions in the coming years – something that would be virtually impossible to do without citizen science.
Enabled by the generous support from the BrightFocus Foundation, the EyesOnALZ development team has launched Stall Catchers - an online game that anyone can play - in October, 2016.
Sources:
-
BrightFocus Alzheimer’s Disease Toolkit: http://www.brightfocus.org/alzheimers/information
-
Schaffer, C. “AD talk” at WeCureALZ all-hands meeting, Cornell University, 2016-03-03.
-
Blennow K, de Leon MJ, Zetterberg H. Alzheimer’s disease. Lancet. 2006;368:387–403.
Try your skills at finding capillary stalls & help accelerate Alzheimer's research in Stall Catchers: stallcatchers.com !
Learn more about how participating in Stall Catchers can help find an Alzheimer's treatment faster in this video: